Identifiers to Enable Pharmaceutical Quality Knowledge Management (PQ KM) – a Progress Report
Version dated: September 15, 2025
Background and Rationale
As pointed out in the 2022 Joint Reflection Paper (JRP), effectively managing changes to pharmaceutical manufacturing processes is challenging, especially to marketing authorization holders (MAH) submitting the same dossier to multiple regions. Different formats and contents in the dossier are expected due to country/region specific conventions and legal/regulatory expectations. Such inconsistencies also cause inefficiency amongst regulators and hamper mutual reliance efforts to share knowledge amongst regulators and facilities to be more nimble, agile in regulatory decision making. The envisioned PQ KM system aims at strengthening international collaboration to support global development, manufacture, and supply of pharmaceutical and biological/biotechnological products.
One key challenge to international collaboration stems from how to identify or confirm that it is the same product under assessment by different national regulatory agencies (NRA) in parallel or at different times. Solving this challenge would allow NRAs to effectively collaborate or share their assessment outcomes and decisions, and eventually make the same product available to the patients in multiple regions sooner. Although some authorities use national or regional identifiers, a common and interoperable limited set of identifiers for manufacturing facilities, pharmaceutical products, substances, marketing applications, and/or marketing application holders is not currently endorsed.
Purpose and Approach
Recognizing the above challenge, the JRP called for a cross-organization collaboration on defining identifiers. Subject matter experts from ICMRA, IPRP, ICH, and PIC/S member agencies were convened to form a working group. The group was tasked with considering the status of current standards, their implementation, potential need for new standards and approaches for the development, adoption, operation, governance, and maintenance of a limited set of internationally harmonized identifiers related to pharmaceutical products and the establishments that manufacture them.
In the development phase, the group focused on key quality information in the eCTD Module 3, and examined current practices by different NRAs (e.g., EMA, HPRA, PMDA and USFDA) and existing standards and initiatives, such as:
- Key organization information and quality attributes to identify the same product
- ISO IDMP standards (11615, 11616, and 11238) in identifying medicinal products, pharmaceutical products and substances
- Substance, Product, Organization and Referential (SPOR) Data Management Services from the EMA
- Global Substance Registration System (G-SRS), which provides common unique identifiers (UNII) for substances
- Pharmaceutical Inspection Co-operation Scheme (PIC/S) recommendation for unique facility identifiers
Additionally, the group verified the selected identifiers using information from global submissions received by two ICMRA Pilots (i.e., Collaborative Hybrid Inspection Pilot (CHIP) and Collaborative Assessment Pilot for Quality/CMC Post-Approval Change (PAC)) launched in 2022. The goal was to determine feasibility of the group’s recommendations by assessing if these identifiers are commonly used in submissions, and to identify potential gaps or challenges in adopting these identifiers.
The above effort resulted in a proposed identifiers framework, which should help NRAs to quickly confirm that the same drug product is under assessment to accelerate or better enable global collaboration and/or mutual reliance.
Outcome and Discussion
This framework, as proposed, contains two tiers of identifiers:
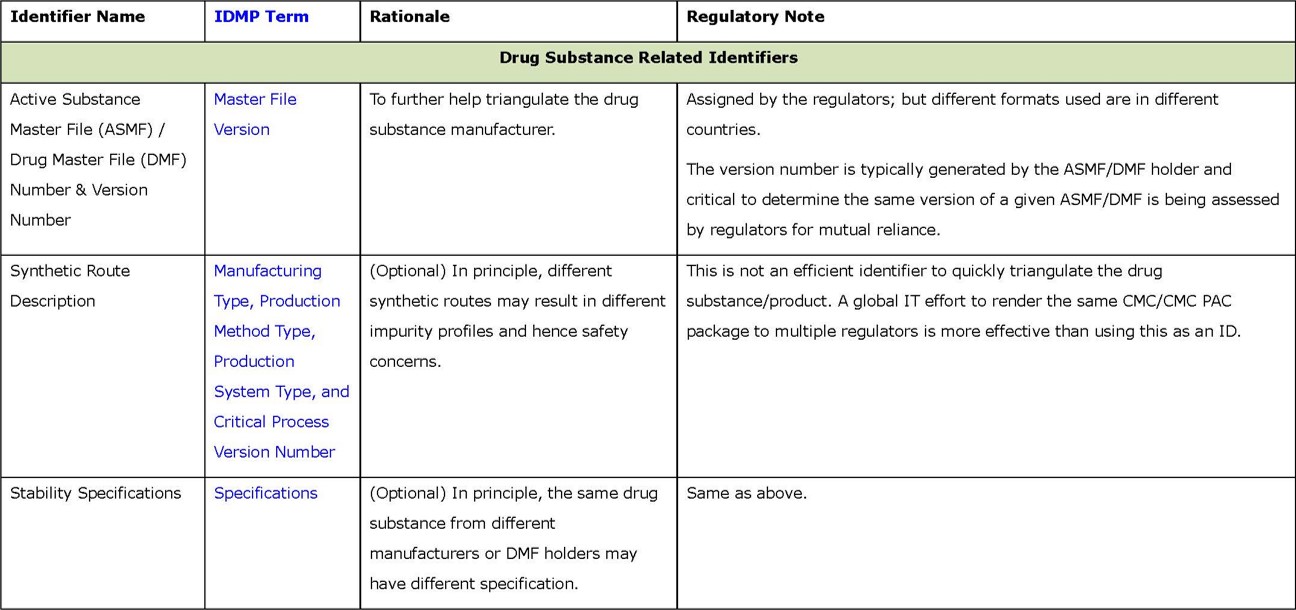
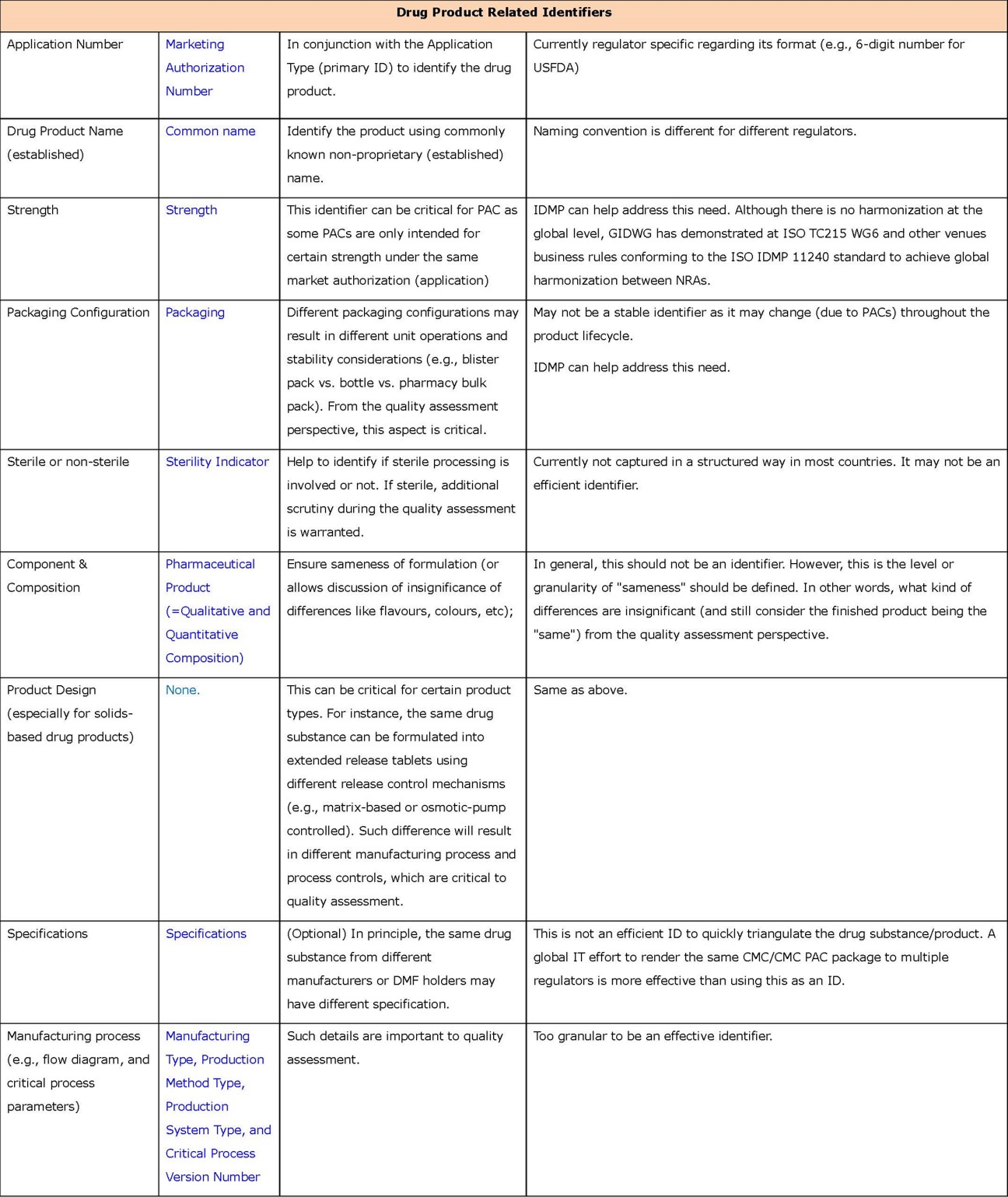
- Primary identifiers (in Appendix 1) are key information about drug substance and product, and associated organizations (e.g., MAH and manufacturers). Such information is currently mandatory and submitted in dossiers, typically using well formulated terms or codes in highly structured format. They can be easily located in submissions (e.g., the cover letter or application form) to serve as effective identifiers. It is estimated that using these primary identifiers collectively should give NRAs high confidence in determining product “sameness”.
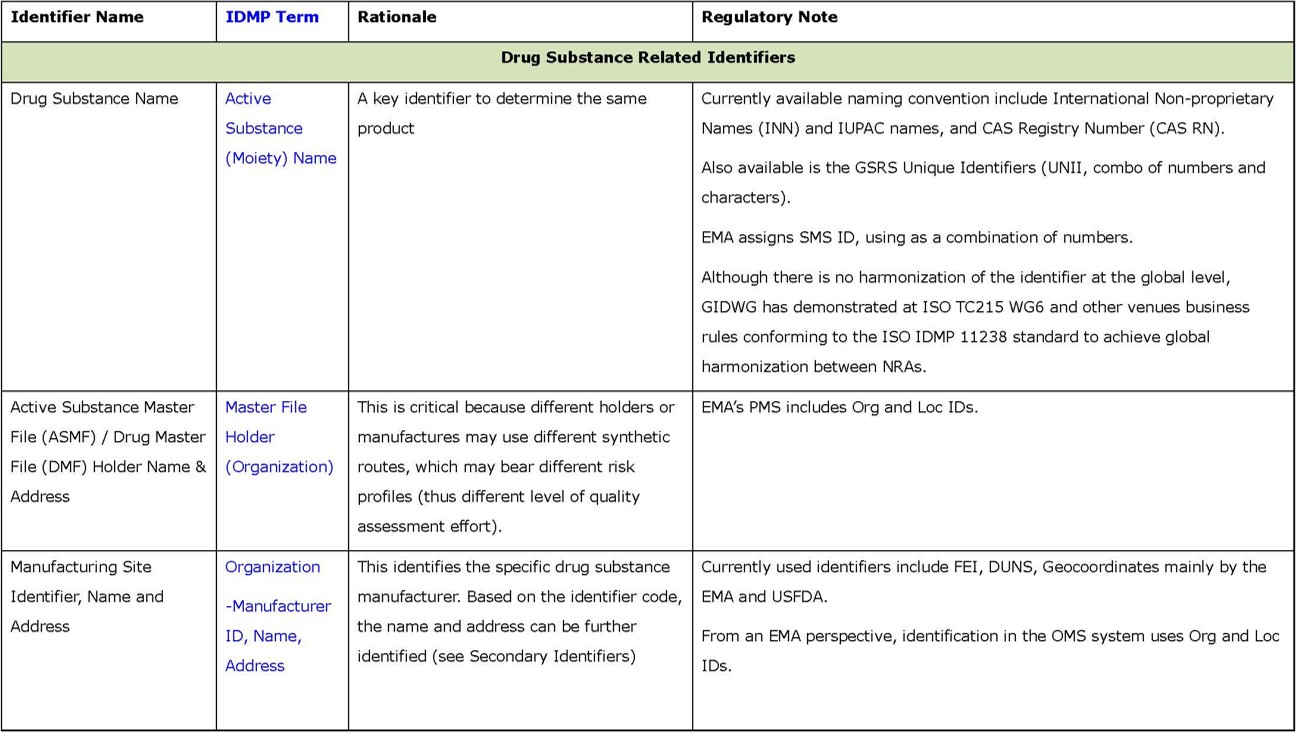
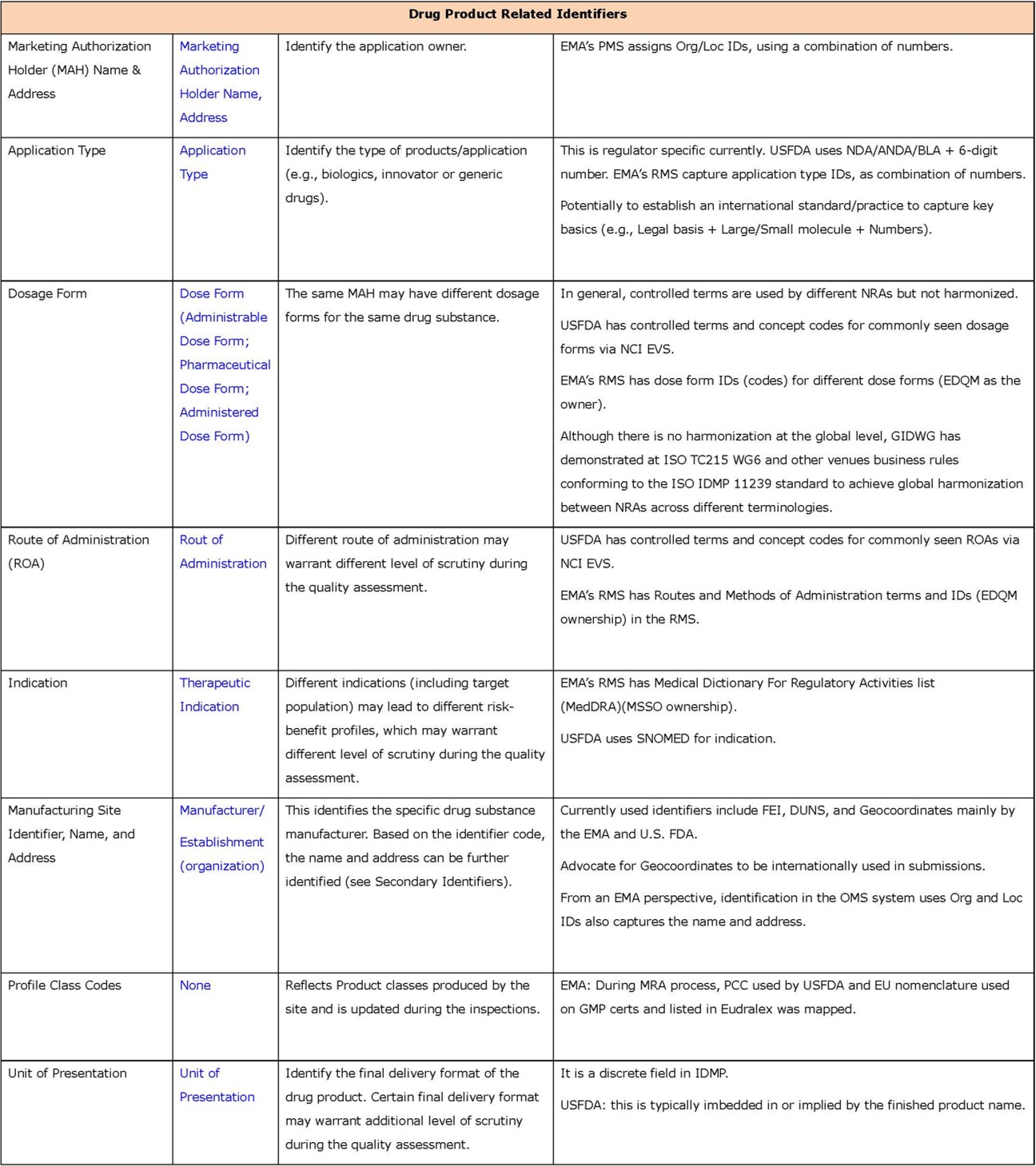
- Secondary identifiers (in Appendix 2) are additional, and contain more granular information about drug substances and products, and associated organizations. Using these additional identifiers should give a higher level of confidence in determining product “sameness”; however, it may take significantly more effort because they are typically text-based and deeply “buried” in submissions. Therefore, they are expected to be less effective as identifiers.
Regarding the primary identifiers, the group has a high level of consensus on what the limited set of primary identifiers should be. Also, a majority of them have been well defined in ISO 11615/11616/11238, as noted in the appendices. In fact, these identifiers have been widely and effectively used by NRAs, following their own conventions and practices, and using different controlled terms and system generated codes. For instance, FEI and DUNS codes are used by the USFDA to identify manufacturing establishments; however, using such codes is not required by PMDA. EU/EEA NRAs and EMA use a system called “Organisation Management Service (OMS)” that supports EU regulatory activities and business processes; it stores master data comprising organisation name and location address for organisations such as MAHs, sponsors, regulatory authorities and manufacturers. For application types, different NRAs have different formats due to specific regional requirements. For dosage form, there is not a internationally agreed-upon list of controlled terms. Consequently, the same product can still be labelled differently using this proposed framework, compromising the interoperability of these identifiers across NRAs. Therefore, successful use of these identifiers for reliance is contingent upon the eventual adoption of internationally recognized and controlled terms or codes.
Through the verification effort referencing the two ICMRA pilots, the group found that 1) most primary identifiers are commonly used in global submissions, and 2) a few secondary identifiers may need to be elevated to primary due to their consistent usage. Below are some key observations.
- Most primary identifiers (i.e., drug substance name, marketing authorization holder name and address, application type, dosage form, route of administration, and indication) are included in global submissions. This demonstrated the feasibility of these primary identifiers.
- Product type (e.g., chemical/small molecule, biological molecule, other) can be another primary identifier or a substitute for Application Type.
- Unit of Presentation is an important primary indicator. Although not a required data field in the ICMRA pilot submission forms, it could be derived from information provided in other data fields (e.g., dosage form).
- Profile Class Code, a primary identifier, was not required in the pilot submission forms; no submissions mentioned this information either. This may suggest that such an identifier is not necessary. However, it is important to acknowledge that certain identifiers may be meaningful or needed only in specific situations.
- Drug Product Name (text based), a secondary identifier, is a required data field in the pilot submission forms. Applicants consistently provided such information. This suggests that it may need to be elevated to primary identifier.
- Application Number, a secondary identifier, appears to be used commonly to identify a product, and may need to be elevated to primary identifier (at least for near future use). However, the data showed tremendous variation in the naming or coding conventions across NRAs.
- Regarding other secondary identifiers, the verification effort showed that they are rarely used.
The group sees the opportunities in ICH and global efforts, to augment the use of these identifiers. The ICH M4Q under revision could clearly specify these identifiers as mandatory for all future submissions and prescribe the desired locations. In addition, the upcoming ICH Structured Product Quality Submission (SPQS) effort can further harmonize submission content (i.e., data elements and format) and the associated conventions (e.g., business rules, controlled terms and coding strategies); for instance, SPQS can prescribe that all the identifiers are captured in a specific section of a well-structured submission form. Their implementation and adoption these ICH guidelines will greatly accelerate the usage of these identifiers. Additionally, the Global IDMP working group (GIDWG), formed in 2021, is leading to establish a framework for global implementation of the ISO IDMP standards and maintenance of global identifiers.
As resources permit, the group suggests a deeper dive into practices and conventions currently used by different NRAs to determine specific differences and potential approaches to harmonize the format/content of these identifiers, to enhance interoperability. The results from this exercise can also serve as a critical reference especially for the upcoming ICH SPQS effort.
Key references:
- The 2022 PQ KMS Joint Reflection Paper
- Considerations on use of unique identifiers to enable reliance through a global regulatory PQ KMS
- EMA PMS ISO IDMP Implementation Guide Chapter 8 Practical example (July 2022)
- ISO 11238: Data elements and structures for unique identification and exchange of regulated information on Substances
- ISO 11239: Data elements and structures for unique identification and exchange of regulated information on pharmaceutical dose forms, units of presentation, routes of administration and packaging
- ISO 11240: Data elements and structures for unique identification and exchange of units of measurement
- ISO 11616: Data elements and structures for unique identification and exchange of regulated pharmaceutical Product information
- ISO 11615: Data elements and structures for unique identification and exchange of regulated medicinal Product information
Appendix 1: Primary Identifiers
Appendix 2: Secondary Identifiers